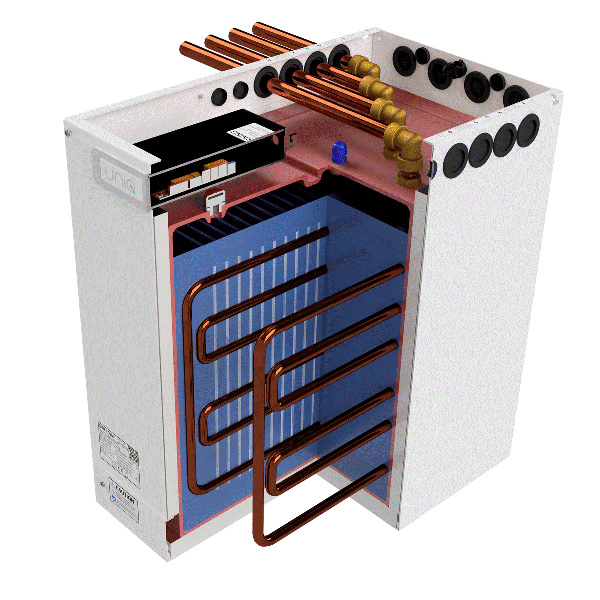
Latent Heat (of fusion)
When a substance undergoes fusion (melting), it absorbs energy from it surrounding to break the intermolecular forces holding the particles together in the sold state. This energy known as the latent heat of fusion, remains stored within the substance, causing it to undergo a change in state with out a change in temperature. An example of latent heat is melting of ice. Even though heat is applied to the ice, the ice’s temperature remains at 0C (32F). And this stays this way until it is completely melted. In other words the Latent heat is the stored energy in a phase change form a solid to a liquid or vice versa.
Sensible Heat
This is the energy used to raise the temperature or reduce the temperature of a give substance. As an example, if we add heat to cold water, we know that the energy will be absorbed and the water temperature will rise. The universal formula for water is that it takes 1 BTU of sensible heat to raise 1 lbs of water 1 degree F.
Scientifically
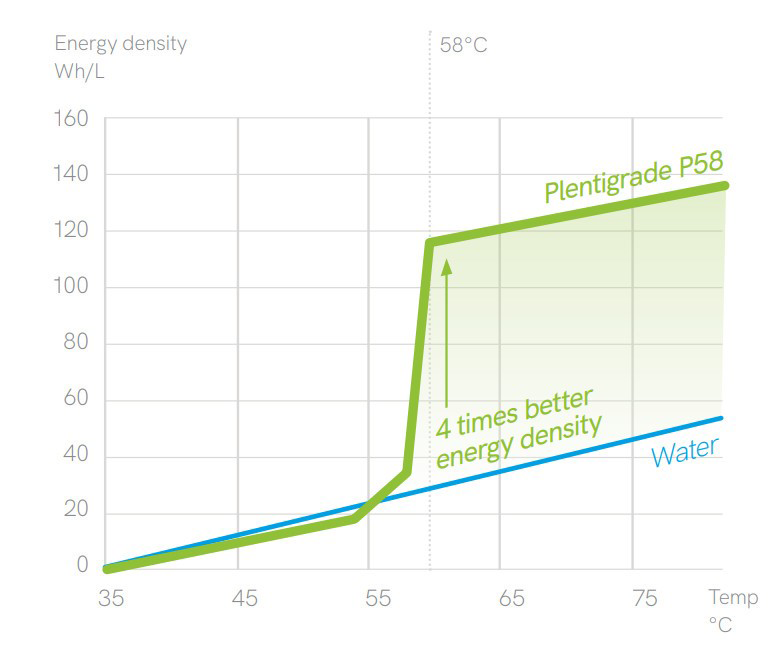
Latent heat can be described as the amount of energy required to change the phase of 1 KG while Sensible heat is the amount of energy required to raise a 1 KG, 1 degree C. The fact is that it takes much more energy to change a phase that to raise the temperature. This is the benefit that a thermal battery takes advantage of.
PCM – Phase Change Material
There are 1000s of different PCM all with different characteristics such as their melting an boiling points. Water is known to melt at 0 C and boil at 100 C under standard atmospheric pressure. However, we know that there are many other materials that melt under different heats such as metals like gold and silver. Wax is a common household material that melts at a certain temperature and similarly salts melt at different temperatures. This is know as its latent heat capacity and will have both a temperature and amount of energy required.
Example: Sensible heat storage Vs. latent heat storage
An 80 gallon tank of water can hold 666.32 lbs of water. We know a BTU is the amount of energy to raise 1 lbs of energy 1 Degree F. So if we store 212F (max temp before boiling) water in the tank we have a maximum theoretical amount of energy of 212-32F (freezing) or 180 degrees of energy in the tank. This means 119,937 BTU of energy. However, we certainly don’t water to be using 32 or 33 degree water so if we like our showers at 110 F then the useable energy is 212-110 or 102degrees. This is 67,964 BTU of stored energy. This is the amount of usable sensible heat energy with out applying extra pressure to the water.
Now let’s say we continue to heat the water until it changes to steam. The steam is still 212 F but we have to use a substantial amount of energy, as a matter of fact it takes 965 Btu to change one pound of water at 212°F to one pound of steam at 212°F. So with our 666.32 Lbs. of water we can store an additional 642,998 BTU of energy plus the sensible energy we calculated earlier of 67,964 BTU. In other words we can store 9.4 times more energy in latent storage that in sensible.